
Experience Innovation, Precision, and Unwavering Commitment
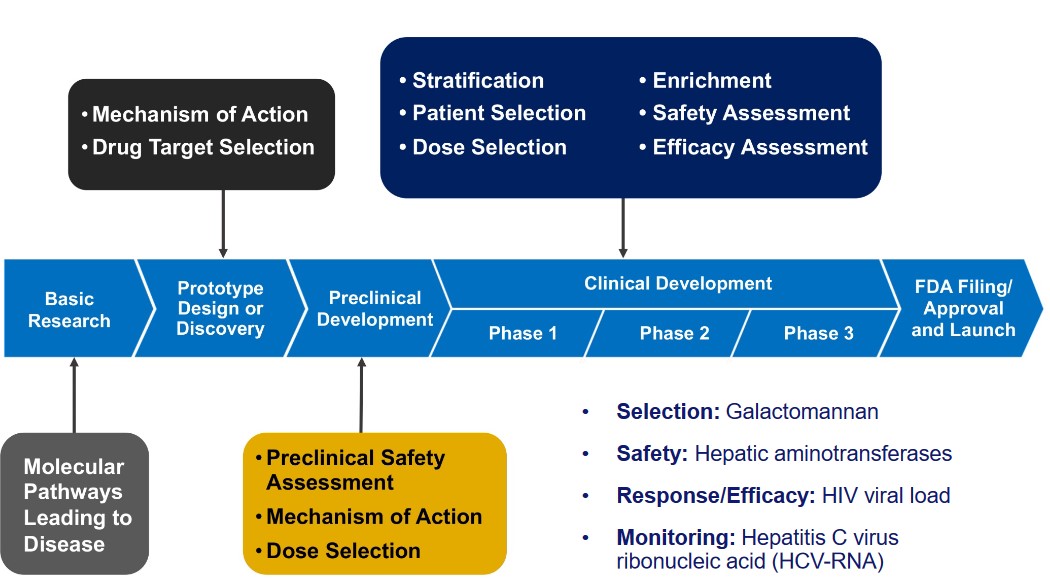
Precision defines our approach to formulation development. With a meticulous attention to detail, following by the FDA development methodology, we navigate the complexities of creating pharmaceutical products, ensuring they adhere to the highest standards of efficacy, safety, and regulatory compliance.
At M&D Health, our commitment to excellence extends beyond research and production. We have honed a spectrum of development competencies that underscore our leadership in the pharmaceutical industry. Our profound understanding of the developmental landscape empowers us to innovate, create, and deliver results that surpass expectations.
- Rat/Mouse PINP EIA
- IDS-iSYS PTH (1-34)
- Corticosterone EIA
- Corticosterone HS
- Urine CartiLaps EIA
- Rat/Mouse PINP EIA
- IDS-iSYS PTH (1-34)
- Corticosterone EIA
- Corticosterone HS
- Urine CartiLaps EIA
FDA & GMP Certified Excellence
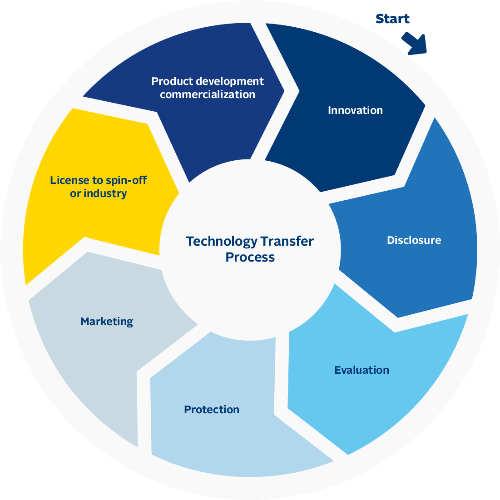
In our esteemed pharmaceutical company, we uphold the pinnacle of industry standards with our meticulously crafted Technology Transfer Process, distinguished by the esteemed FDA and GMP certifications. Guided by a team of seasoned professionals, including accomplished doctors and research experts, we meticulously document and transfer cutting-edge technologies and research insights. Our ultimate mission is to expedite the development and distribution of life-altering medications, propelling us closer to a world of enhanced well-being and prosperity.
Stability and Consistency
Our legacy of over 25 years underscores our commitment to stability and consistency. Clients entrust us with their most critical projects, secure in the knowledge that our team is unwavering in its pursuit of perfection.
One size rarely fits all. Our expertise in customization allows us to craft bespoke formulations tailored to the specific needs of our discerning clientele. Each project receives the attention it deserves, resulting in products that align seamlessly with client objectives.


